Joint Registration and Only Representative
The aim of joint registration is to decrease the cost of registration by splitting the fee etc. and reduce the number of animal testing.Lead registrants, who are appointed by the SIEF members for each substance, prepare and submit the joint registration dossier on behalf of the SIEF. TMGD TR can assume the role of chief registrar upon your request or manages leading companies and consortia as your representative. The main goal is to minimize your cost Having managed approximately 1800 registration dossiers including 200 lead registrations in EU REACH, TMGD TR is the holder of data for many substances and thus, we offer you the advantage of having a much faster & cost-effective registration process for the SIEFs that we are the lead of.
KKDIK Timeline consist of 3 main phases:
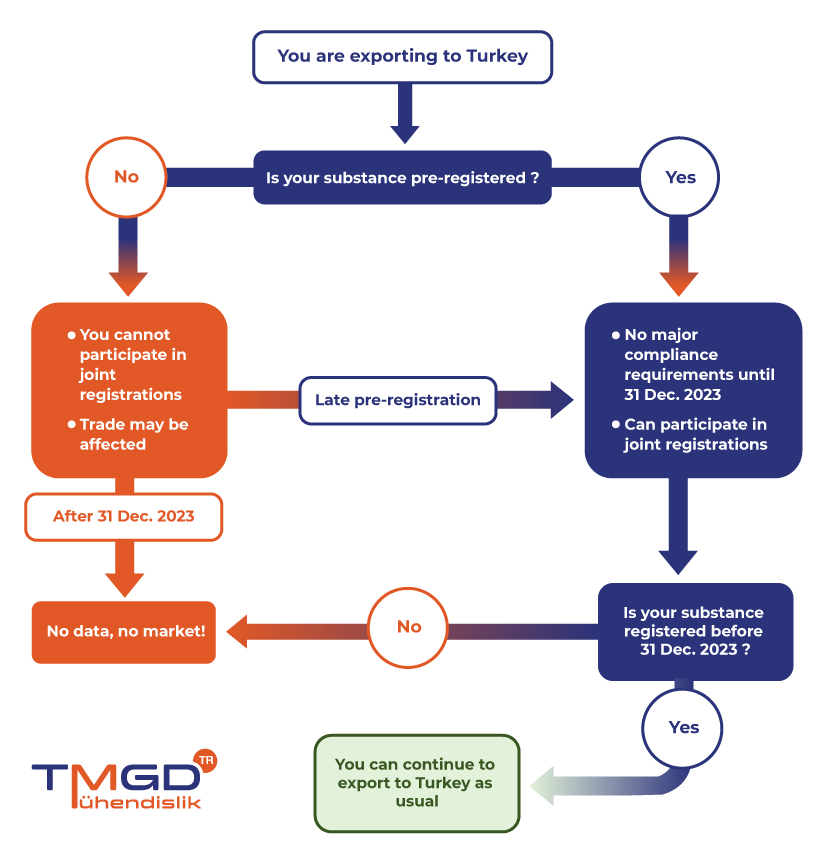
- Pre-registration (The application deadline has passed but the system is open and pre-entry is possible)
- Registration (Deadline 31st Dec 2023)
- Post-registration (As of January 1, 2024, those who do not register will not be able to export goods to Turkey)
Time for Turkey Reach (KKDIK) Registrations is about to expire!
It is strongly recommended for exporters to Turkey to submit late pre-registrations (LPR) as soon as possible to secure business if not done already before 31st December 2020. Pre-registration carries crucial importance to be able to actively participate in SIEF activities for joint registrations.
KKDIK allows only pre-registered substances to be placed in the Turkish market from the 1st of January 2021 and onwards until the deadline of registration, 31st of December 2023. Pre-registration is a simple activity without major compliance requirements that also allows TMGD TR to represent your best interest within the SIEFs.
KKDIK is a comprehensive regulation. However, some substances are exempted from registration either because they are covered by specific regulations such as radioactive waste or regarded as less risky such as polymers. The registration process is not the same for every substance for instance under some conditions, registration of on-site or transported isolated intermediates is relatively easy. You can find information about exempted substances on our website or you can e-mail us at info@tmgdmuhendislik.com to find out more about the registration process and exempted substances.
Registration requirements change depending on exported tonnage of substance. When the tonnage increases, additional information is needed. For instance, when the tonnage of substance is 10-100 tons per annum, chemical safety report should be provided. Therefore, the best and easiest action would be e-mailing us your substance details and we will provide you your customized complete obligations under Turkey REACH. Now you can fill out a form and let us contact you. There are 28 Chemical Engineers in our team
On June 23, 2017 the Turkish Ministry of Environment and Urbanization (MoEU) published a regulation named Kimyasalların Kaydı, Değerlendirilmesi, İzni ve Kısıtlanması Hakkında Yönetmelik (Bylaw on Registration, Evaluation, Authorization and Restirction of Chemicals) on Official Gazette Rep. No: 30105 for the management of substances, very similar to the EU REACH
Regulation, (EC) No 1907/2006.
The KKDIK regulation came into effect on December 23, 2017.
KKDIK covers manufacturing, placing on the market or use of the substances on their own, in a mixture or in an article and placing the mixtures on the market unless they are:
- Wastes
- Radioactive materials and wastes
- Substances used in defense industry
- Non-isolated intermediates
- Substances under transport and subject to customs
- Substances in freezones or temporary storage waiting to be re-exported
To regulate the administrative and technical procedures and principles regarding the registration, evaluation, authorization and restriction of
To ensure a high level of protection of human health and the
To promote use of alternative methods for assessment of hazards of substances while enhancing competitiveness and
All substances that are in scope of KKDIK have to be registered and pre-registered if they are produced or imported more than 1 ton per year unless they are exempt. Substances that are not pre-registered until the deadline will not be allowed to be placed in the Turkish market until their registration is complete. Substances that are not registered in time will not be allowed to be placed in the market
- Manufacturers
- Importers
- Non-Turkey manufacturers can appoint an “Only Representative (OR)” similar to EU REACH for the pre- registration and registration
Pre-registration within the scope of KKDIK had to be made by 31 December 2020. Since the system is not closed, pre-registration and master registration can be done together. The deadline for this is 31.12.2023. The registration date is not affected by the tonnage band.
A natural person or legal entity established physically in TURKEY Equipped with sufficient knowledge in the practical handling of the substances and information related to them (CICR/KEK, CLP/SEA, SDS/GBF, TURKEY-REACH/KKDIK, BPR, PPPR, related TR )
Appointed by a mutual agreement with a manufacturer, formulator or article producer, established outside Turkey Responsible for complying with the legal requirements for importers under TURKEY-REACH (KKDIK)
Only representatives can represent more than one non-TR supplier, but must keep the information related to each of them. The non-TR company has to inform the importer (s) within the same supply chain of your appointment as an only These importers are then regarded as downstream users for TURKEY-REACH.